Clinical response of advanced renal carcinoma’s patients treated with small immunomodulatory proteolipid particle
Caballero Aguirrechu 1, C. Mesa 2, R. Alvarez 2, L. Oliver 2, D. Gomez 1, R. Rodriguez 1, A. Rosales 1, G. Vega 1
(1) “Hermanos Ameijeiras” Hospital, La Habana – Cuba, (2) Molecular Inmunology Center, La Habana – Cuba
Objective:
Renal cancer is a tumor with high immunogenicity and resistance to systemic chemotherapy; in the metastatic condition, surgery alone does not show high survival rates. The main therapeutic strategies are directed at molecular targets. Objectives: Characterize patients according to clinical-epidemiological data, risk group, survival and safety of the treatment administered, as well as the kinetics of peripheral myeloid populations.
Methods:
A descriptive, retrospective, cross-sectional study was carried out with 30 patients with metastatic or recurrent renal cell carcinoma who received treatment with very small proteolipid particle immunomodulator of the tumour microenvironment (natural NAcetyl GM3/VSSP). Survival was estimated with Kaplan Meyer curves, clinical benefit analysis (p=0.05) with non-parametric methods.
Results:
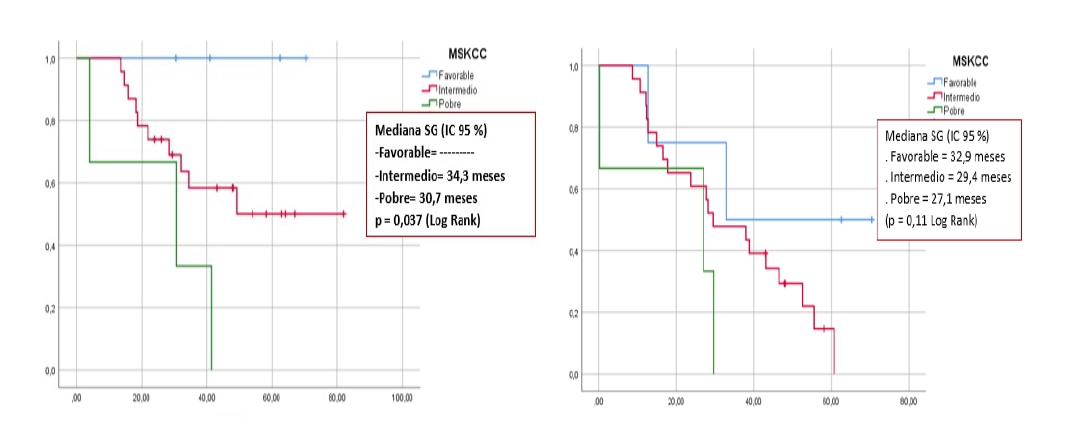
The average age was 62 years, the male sex predominated (76.7%), the presence of lung metastases (60%), and tumour in the right kidney (40%). The median progression-free survival was 42.6 months and overall survival was not reached, with a better response for patients in the favourable and intermediate risk groups and those who received more than 5 doses of VSSP. No grade 3-4 adverse events were reported.
Conclusions:
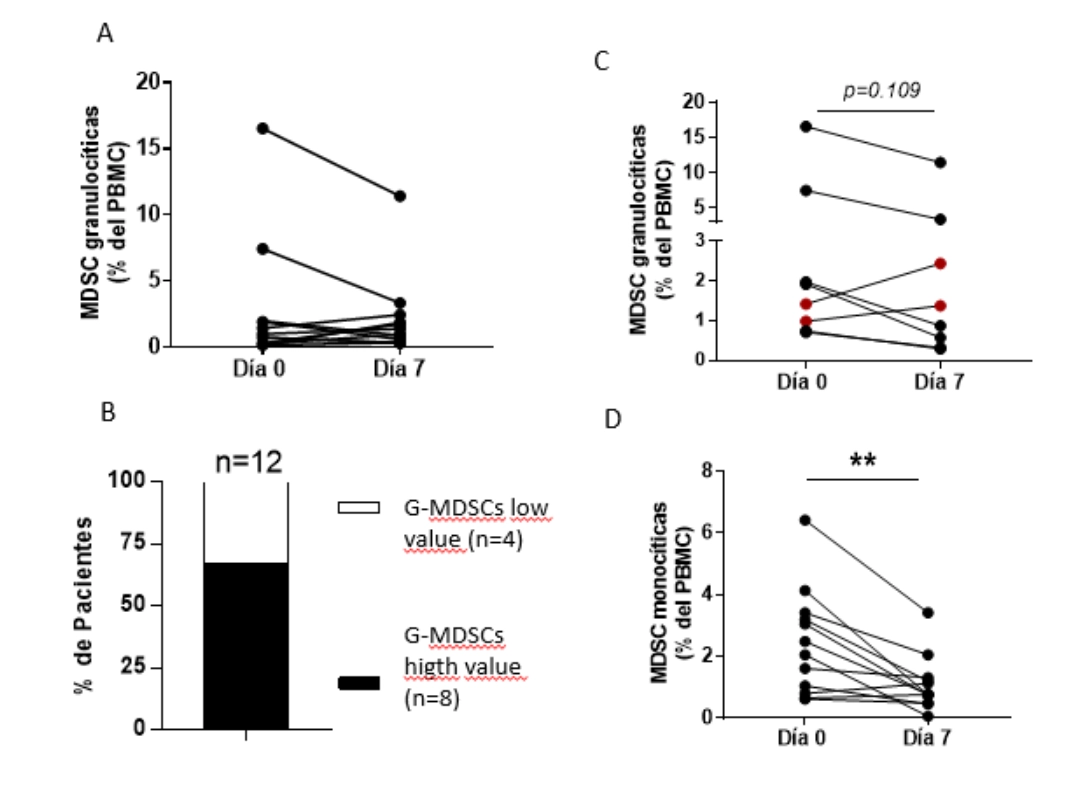
Young patients with CRC with lung metastases, from the intermediate risk group and with tumor in the right kidney predominated. Survival rates with the use of natural VSSP correspond to those reported with other immunotherapy drugs in this setting and the administration of the product was safe. Myeloid cell population determinations were elevated at inclusion and their decrease was observed with the administration of VSSP.
Resulted:
Fig 1. Serological peripheral myeloid cell’s kinetics in patients with metastatic renal carcinoma.
OS risk group
PFS risk group
Fig 2. OS and PFS according to MSKCC risk groups (Kaplan-Meyer)